Carbon Black
Carbon Black is the oldest man-made nanomaterial. Simple methods of producing soot, used to make ink, were already known in ancient China and Egypt. Soot was then obtained by burning oil or resin under suspended porcelain dishes. The importance of carbon black has been systematically growing since the dissemination of printing, while the major breakthrough came after the discovery that adding carbon black to rubber significantly improves its mechanical properties. This fact contributed to the development of the automotive industry, enabling the production of tires with a durability of tens of thousands of kilometers. Currently, carbon black is an industrial raw material, primarily as a filler in the production of tires and other rubber materials, and as a pigment in the production of paints, varnishes and plastics. However, as a nanomaterial, carbon black has remained underestimated. One of the few applications of carbon black that effectively uses the possibilities resulting from the nanosize is the role of a carrier of catalytic nanoparticles, which can be evenly dispersed on its highly developed surface, which allows to significantly increase the efficiency of the applied catalyst, while reducing its consumption and cost. Catalytic nanoparticles are defined as catalyst particles with a size ranging from 1 to 20 nm. The importance of processes using catalysts is growing dynamically, especially in the field of issues related to energy storage, obtaining and conversion, i.e. key issues for the quality of human life and the further development of civilization.
Carbon Black is created as a result of burning carbon-containing materials with insufficient access to oxygen. Carbon black, produced in industrial conditions (technical carbon black, in short called carbon black) is obtained under strictly defined, repeatable conditions. The reaction parameters are set so as to obtain a product with the expected properties and in a satisfactory amount, which fundamentally distinguishes it from soot from chimneys or internal combustion engines. The types of carbon black can differ significantly in the shape of aggregates, shape, size and method of combining primary particles and their internal structure (organization of carbon layers). These differences result from many reasons, the most important of which are the type (chemical structure) of the precursors and the conditions of formation (especially temperature and reaction time). Several methods are used to produce carbon black. In the kiln method, currently the most common, the production process is carried out with a strictly defined supply of oxygen. The raw material is mineral oil with an admixture of combustible gas, whose task is to maintain the required temperature (1200-1500 ºC). The choice of process parameters allows to obtain carbon black with particle diameters from about 15 nm to almost 80 nm. One of the oldest methods of carbon black production is the flame method. Liquid or molten raw materials are burned in a cast iron flat tank with one flame. The air supply is regulated by the side intake slots. Combustion products, after passing through the chimney channel, are directed to chambers with cooled walls or to a system of cyclones and filters in order to capture soot. The soot obtained consists of large particles with a diameter of 120 to 200 nm. In the production of carbon black by the gas method, combustion takes place in an open system. Vapors of coal tar distillates burn with free air access. The soot settles on the walls of the rotating drums (filled with cold water) located above the burners. This production method produces particles that are uniform in size, which can be adjusted between 10 and 30 nm. Thermal carbon black production is now rarely used and limited to specialized applications. Combustion takes place in a closed system. Due to the low temperature (approx. 1300 ºC) and long reaction time, very large particles (with a diameter of up to 500 nm) and a small specific surface area (a few m2/g) are obtained. Acetylene soot is formed as a result of thermal decomposition of liquid and gaseous hydrocarbons, without access to air. It is the only soot produced by a strongly exothermic reaction. The presence of long, parallel carbon layers is characteristic of acetylene black. High reaction temperature (approx. 3000 ºC) and high pressure (over 100 Pa) cause that the obtained particles are of irregular shape, characterized by a high degree of crystallization and high electrical conductivity.
The specific surface area of carbon black depends on the forming conditions and varies in a wide range, from several to several hundred m2/g. Formation conditions also affect the structure of the carbon black aggregates and primary particles. Carbon blacks with a low surface area (less than 50 m2/g) are made of large particles (over 40 nm in diameter). These are grades of carbon black formed at a relatively low temperature, with a long forming time. Carbon black grades with a higher specific surface area, but not exceeding 400 m2/g, are made of fine particles (approx. 10-40 nm in diameter). The process of forming these types of carbon blacks is faster and takes place at a higher temperature than carbon blacks with a low specific surface area. The high value of the specific surface area of other types of carbon blacks (above 400 m2/g) is the result of a different nature of the manufacturing process (exothermic process) and/or additional processes to which these carbon blacks are subjected and is not directly related to the particle diameter. Some types of carbon black are subjected to the oxidation process in appropriately set conditions, which affects the change in the chemical and application properties of the obtained materials.
Carbon Black has the form of three-dimensional aggregates, consisting of multiple branched chains of complex shape. Soot aggregates consist of spherical primary particles with a diameter of less than 100 nm, which in turn are made of concentric carbon layers, parallel to each other and approximately 0.335 nm apart. Thanks to this characteristic, multi-scale organization, they can be clearly distinguished from other solid carbon particles when observed in a Transmission Electron Microscope (TEM).
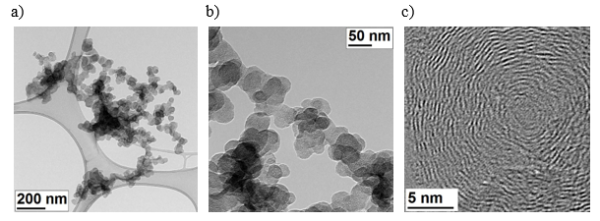
Fig. Multiscale organization of soot structure: soot (joined to carbon film) (a), enlarged his fragment, consisting of spherical primary particles (b), magnification of part of primary particle, built with concentric stacked layers of carbon (c)